Oxygen is everywhere in the Earth’s atmosphere, which is essential for life and a key factor driving the development of many industries. As a core property of oxygen, its density profoundly impacts various fields, from basic industries to advanced scientific research, encompassing a complex and vast knowledge system with significant application potential.
Under standard conditions (0°C or 32°F and 101.325 kPa), the density of oxygen is 1.429 g/L, which is equivalent to 0.001429 kg/L or 1429 kg/m³.
Oxygen Density
Under standard conditions—0°C (32°F) and standard atmospheric pressure (101.325 kPa)—the density of oxygen is 1.429 g/L. Converting to other units, such as g/cm³, kg/L, and kg/m³, the density of oxygen at standard conditions is 0.001429 kg/L or 1429 kg/m³. According to the ideal gas law, oxygen density can be calculated using the formula:
Density = Molar Mass of Gas × Pressure of Gas / (Gas Constant × Temperature of Gas).
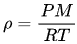
Why is Oxygen Density Important?
Oxygen density refers to the number of oxygen molecules contained in a unit volume under specific conditions (usually associated with temperature and pressure). Understanding oxygen density is crucial in many fields. In gas preparation and storage, accurate control of oxygen density optimizes container design and ensures transportation safety. In scientific research, whether in physical chemistry experiments or atmospheric science exploration, oxygen density is a key parameter. In medical emergencies, precise oxygen delivery depends on controlling its density to ensure the patient inhales the correct concentration and amount of oxygen.
As a critical component of Earth’s atmosphere, oxygen makes up about 21% of the air by volume. When handling mixed gases, knowing the oxygen density is essential for calculating the overall density of the mixture and the proportion of each component. Therefore, changes in oxygen density under different pressure and temperature conditions should not be overlooked.
- Industrial Sector: In steel smelting, precise oxygen density control improves smelting efficiency and ensures product quality. In chemical synthesis, where oxygen is a reactant, its density affects the reaction process and product yield.
- Healthcare: Hospitals’ oxygen supply systems, including high-pressure oxygen chambers, rely on accurate oxygen density control to deliver the right amount of oxygen to patients.
- Environmental Ecology: Changes in atmospheric oxygen density reflect the health of ecosystems, playing a vital role in the study of climate change and biodiversity.
Researchers are increasingly focusing on the profound impact of oxygen density on various industries and daily life.
How to Measure Oxygen Density
The standard procedure for measuring oxygen density is usually set by international standardization organizations and national institutions. In the U.S., standards are often issued by the American National Standards Institute (ANSI) or the National Institute of Standards and Technology (NIST), while in Europe, they are primarily set by the European Committee for Standardization (CEN).
These organizations have established standardized testing procedures based on advanced scientific and engineering practices to obtain precise data. The authority of the results comes from the strict control of measurement variables and the use of high-precision measuring instruments.
Historically, scientists made significant efforts to measure oxygen density. In the 18th century, chemists like Lavoisier explored the properties of oxygen while studying combustion and indirectly estimated its density. With technological advancements, today’s gas density sensors allow rapid and direct measurement of oxygen density, and mathematical models can accurately calculate its density at specific temperatures and pressures.
Factors Affecting Oxygen Density
Several key factors influence oxygen density:
Temperature
According to Charles’s Law, the kinetic energy of gas molecules is proportional to temperature. As the temperature rises, oxygen molecules move more vigorously, increasing the distance between molecules and decreasing the density. For example, in high-temperature industrial furnaces, the oxygen density in the surrounding air is lower than under standard conditions.
Pressure
As pressure increases, the distance between oxygen molecules decreases, causing the density to rise. For instance, in high-pressure oxygen tanks, the gas is compressed, resulting in a much higher density than at standard atmospheric pressure.
Purity
Generally, the higher the purity of oxygen, the closer its density will be to the theoretical value. The presence of other gas impurities will cause the average density of the mixture to deviate from pure oxygen density.
Humidity
Water vapor dilutes oxygen, lowering its density. In a humid environment, the actual mass of oxygen in the same volume of air is less than in dry air.
The following are examples based on the ideal gas equation for reference only.Formula:
| Sample Number | Temperature (℃) | Pressure (kPa) | Oxygen Purity (%) | Relative Humidity (%) | Oxygen Density (g/L) |
| 1 | -20 | 101.325 | 99 | 10 | 1.410 |
| 2 | 0 | 101.325 | 95 | 20 | 1.343 |
| 3 | 20 | 101.325 | 90 | 30 | 1.262 |
| 4 | 40 | 101.325 | 85 | 40 | 1.169 |
| 5 | 60 | 101.325 | 80 | 50 | 1.062 |
| 6 | 20 | 80 | 95 | 20 | 1.127 |
| 7 | 20 | 120 | 95 | 20 | 1.776 |
| 8 | 20 | 101.325 | 70 | 20 | 0.999 |
| 9 | 20 | 101.325 | 95 | 60 | 1.305 |
| 10 | 20 | 101.325 | 95 | 80 | 1.267 |
Properties of Oxygen
Chemical Properties
Oxygen is chemically reactive and highly oxidizing. At room temperature, it can react with many substances. For example, the rusting of iron is a slow oxidation process involving iron, oxygen, and water. Combustion is a more rapid oxidation reaction where oxygen supports the burning of flammable materials, releasing a large amount of energy.
Physical Properties
Oxygen is a colorless, odorless, and tasteless gas at standard temperature and pressure. Its density is slightly higher than that of air, and it is slightly soluble in water. Under standard atmospheric pressure, when cooled to -183°C, oxygen turns into a pale blue liquid. Further cooling to -218.8°C will cause it to solidify into a pale blue snow-like solid.
Biological Function
Oxygen is essential for life for almost all living organisms on Earth. Through respiration, organisms inhale oxygen, which participates in energy metabolism within cells, breaking down organic matter and releasing energy required for growth, movement, reproduction, and other physiological activities.
Uses of Oxygen
Steel Smelting
In steelmaking, high-purity oxygen is blown into molten iron to oxidize impurities such as carbon, sulfur, and phosphorus, which are released as gases, effectively reducing impurity levels and improving the quality of the steel.
Chemical Synthesis
Oxygen is widely used in chemical processes as a reactant or oxidizing agent. For example, in the synthesis of ethylene oxide, ethylene and oxygen react in the presence of a catalyst to produce ethylene oxide, which is used to make polyester fibers and many other chemical products. In sulfuric acid production, oxygen is used to oxidize sulfur or sulfur compounds to produce sulfur dioxide, which is further oxidized to form sulfuric acid.
Healthcare
Oxygen is used in hospitals in emergency rooms, patient wards, and operating rooms to provide life-saving oxygen to patients with compromised lung function or difficulty breathing. Hyperbaric oxygen chambers are used to treat carbon monoxide poisoning, anaerobic infections, and decompression sickness.

Wastewater Treatment
Oxygen is injected into wastewater to provide an essential environment for aerobic microorganisms, which decompose organic matter in the water, helping to purify the water and meet discharge standards.
Aerospace
In high-altitude flight, where the air is thin, aircraft oxygen systems control the supply of oxygen to the cabin and cockpit based on oxygen density. Liquid oxygen is also used in rocket fuel, where it combines with liquid hydrogen or other fuels to provide the necessary thrust for rocket launches.
Aquaculture
Dissolved oxygen levels in water are a critical indicator in aquaculture. Adequate oxygen supply ensures the respiratory needs of fish and other aquatic organisms, supporting their growth and reproduction. Oxygen is added to aquaculture systems through aerators and oxygenation devices, optimized based on oxygen density and solubility principles.
Energy Sector
In emerging fuel cell technologies, oxygen is used as the cathode reactant in electrochemical reactions with anode fuel, directly converting chemical energy into electrical energy. This clean energy source powers portable electronics, electric vehicles, and more.
Liquid Oxygen and Cryogenic Technology
When the temperature drops below -183°C, oxygen becomes a liquid, known as liquid oxygen. It is pale blue and highly oxidizing. According to thermodynamics, when liquid oxygen comes into contact with objects at temperatures above its boiling point, it rapidly evaporates, absorbing large amounts of heat and achieving efficient cooling.
However, storing liquid oxygen presents challenges. It requires maintaining a low-temperature environment to prevent vaporization and a sharp increase in pressure. Additionally, liquid oxygen can react violently with organic materials, even causing explosions. Therefore, storage containers must be well-insulated and designed to prevent explosions, often using double-walled vacuum insulation.
How to Obtain Oxygen
Cryogenic Air Separation
This process separates oxygen from air by taking advantage of the different boiling points of nitrogen and oxygen. Air is compressed, cooled, and liquefied, then distilled through a distillation column. Due to nitrogen’s lower boiling point, it vaporizes first, while oxygen remains at the bottom of the column. This process yields high-purity liquid oxygen and is the dominant method for large-scale industrial oxygen production.
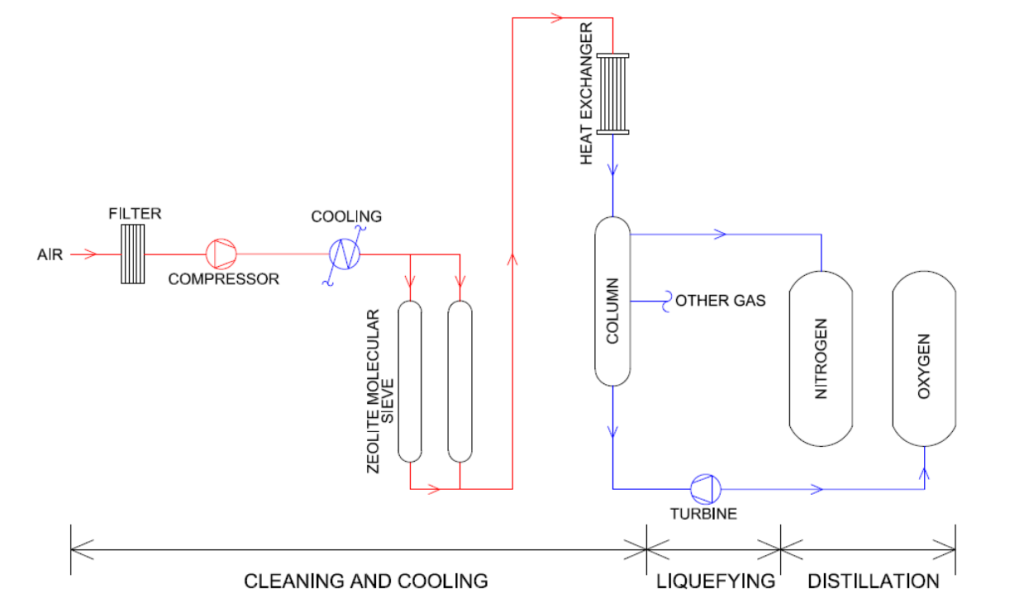
Pressure Swing Adsorption (PSA)
Air passes through a bed of molecular sieves at specific pressure conditions. The sieve adsorbs nitrogen, carbon dioxide, and other impurities more strongly than oxygen, resulting in an oxygen-rich stream. PSA systems are typically used for medium-scale oxygen generation, offering a simple setup with fast startup.
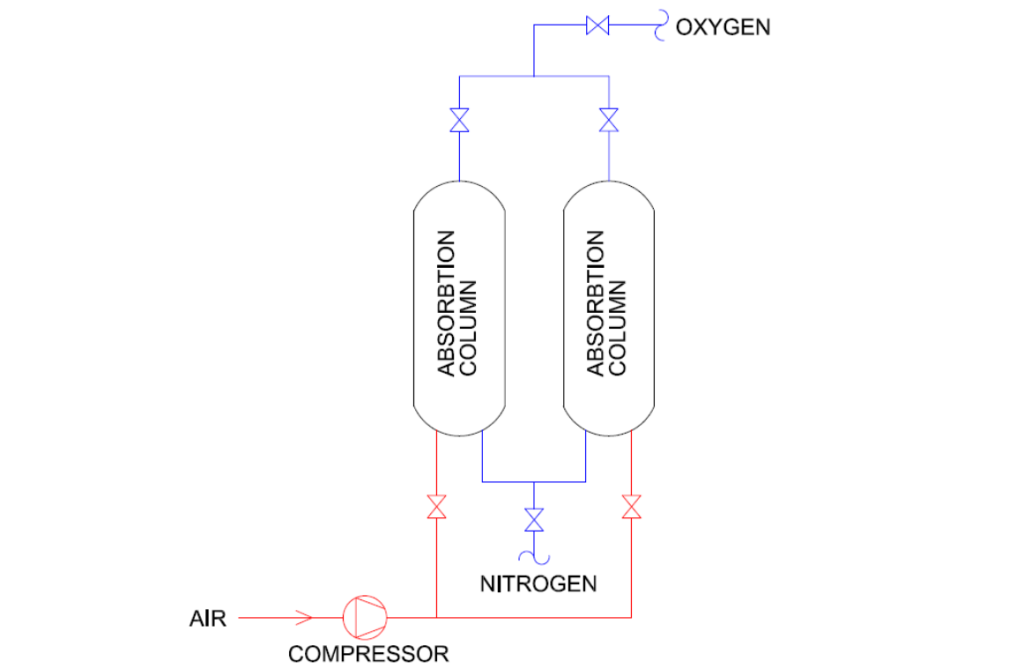
Membrane Separation
Compressed air passes through a membrane with selective permeability. Oxygen molecules pass through the membrane more quickly than other gases, resulting in an oxygen-rich side. This method is simple, energy-efficient, and suitable for small-scale oxygen generation or applications where high purity is not required.
How to Store Oxygen
Oxygen is typically stored in high-pressure cylinders made of alloy steel, which can withstand high pressure. The storage temperature must be carefully controlled because high temperatures can cause pressure to rise, posing safety risks. Cylinders should be stored in cool, well-ventilated areas, away from heat and fire sources. Liquid oxygen is stored in specially designed cryogenic tanks to maintain a low-temperature environment, with safety valves and pressure gauges to monitor and ensure storage safety.

Is Oxygen Dangerous?
While oxygen is vital for life, it can be hazardous if used improperly. Prolonged exposure to high concentrations of oxygen can lead to oxygen toxicity, which may cause lung damage, vision issues, or even convulsions. Oxygen in its liquid form is highly volatile and can cause severe burns upon contact with skin. Therefore, safety precautions must be strictly followed when handling oxygen, especially in industrial and medical environments.
The Future Potential of Oxygen
In the future, oxygen will play a key role in several advanced fields. In space exploration, efficient oxygen production and recycling will support long-term astronaut survival and extraterrestrial base construction. In addressing climate change, new photocatalytic technologies using oxygen may help convert and fix greenhouse gases like carbon dioxide, aiding carbon reduction. In life sciences, oxygen’s role in regulating cell metabolism and immune function will lead to new disease treatments. In short, harnessing oxygen’s potential will drive continuous progress for humanity.
Conclusion
In conclusion, oxygen density is not just a physical parameter but also a link that connects various fields of human development.
As a professional oxygen equipment supplier with over 30 years of manufacturing experience, MINNUO is here to provide you with gas solutions—feel free to contact us for more information!






 sales2:+86 17506119168
sales2:+86 17506119168

