In both industry and research, liquid nitrogen and nitrogen gas are extensively used. However, understanding the differences between these two forms is crucial for selecting the right material for specific needs.
Liquid nitrogen and nitrogen gas are widely used in industry and research. Liquid nitrogen is the liquid form of nitrogen gas, suitable for ultra-low temperature needs. Nitrogen gas, on the other hand, is gaseous at room temperature and is used as an industrial inert gas. Understanding their differences and applications helps in choosing the appropriate material.
This article provides a detailed comparison between liquid nitrogen and nitrogen gas, helping scholars and those in need make informed decisions.
What is Nitrogen?
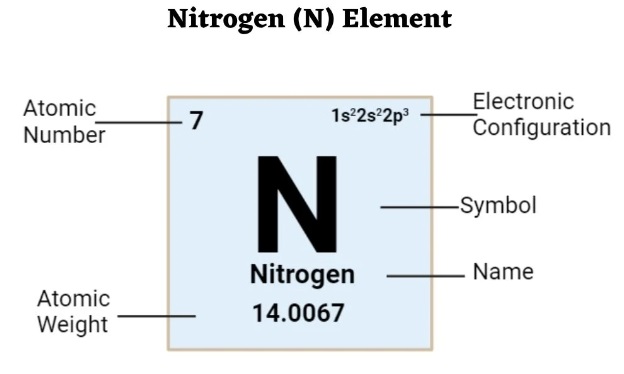
Nitrogen, the most abundant element in the Earth’s atmosphere, makes up 78% of the air we breathe. It plays a crucial role in our daily lives and is widely used across various fields. Understanding the basic properties of nitrogen is fundamental to further exploring liquid nitrogen and nitrogen gas.
- Definition:
Nitrogen is the most abundant element in the Earth’s atmosphere, comprising 78% of its volume.
- Physical Properties:
Nitrogen is a colorless, odorless gas with stable chemical properties.
- Application:
Nitrogen is widely used in the chemical, food, and pharmaceutical industries.
What is Liquid Nitrogen?
Liquid nitrogen is nitrogen in its liquid state, formed by cooling nitrogen gas below its boiling point. This extremely low-temperature liquid has a broad range of industrial and scientific applications.
Definition:
Liquid nitrogen is nitrogen that has transitioned from its natural gaseous state to a liquid state.
Production:
The production of liquid nitrogen involves cooling nitrogen gas to below its boiling point, typically achieved through air separation and liquefaction processes, which require high pressure and low temperatures to produce liquid nitrogen on an industrial scale.
Precautions for the Use of liquid nitrogen
what is the temperature of liquid nitrogen?
The temperature of liquid nitrogen: extremely cold, close to absolute zero
Under normal pressure, liquid nitrogen temperature drops as low as -196℃ (boiling point), and when cooled to -209.8℃, it solidifies into a snow-like solid, ,This extremely low temperature stems from its physical properties – liquid nitrogen is the liquid form of nitrogen gas, colorless, odorless, non-flammable and inert, accounting for 78% of the air volume.
The extremely cold and vaporization expansion characteristics of liquid nitrogen pose potential risks. The following norms must be strictly followed during operation:
Measures to prevent frostbite
Skin contact: Direct contact lasting more than 2 seconds can cause irreversible frostbite. When operating, it is necessary to wear cold-proof gloves, goggles/face masks, long-sleeved clothing and full-coverage shoes. When performing a large number of operations, it is necessary to wear cold-proof clothing and a full-face shield.
First aid: In case of frostbite, immediately soak in warm water at 38 to 42 degrees Celsius for rewarming (do not rub or use hot water), and seek medical attention.
Prevention and control of asphyxia risks
After vaporization, the volume of liquid nitrogen expands approximately 180 times. One cubic meter of liquid nitrogen can be converted into 696 cubic meters of nitrogen gas, rapidly reducing the concentration of oxygen.
Operations must be carried out in well-ventilated open areas. Confined Spaces may cause oxygen deficiency and asphyxia. The operation area should be equipped with oxygen concentration monitors. When the concentration is lower than 18%, positive pressure breathing apparatus should be worn.
Container and Pressure Management
Do not use sealed containers: The vaporization of liquid nitrogen may generate high pressure, which could cause a physical explosion. The vacuum layer and pressure relief device (safety valve, rupture disc) of the storage tank need to be inspected regularly.
Transportation regulations: Handle with care, avoid collision; When placing the storage tank, keep it upright and fixed, away from heat sources and flammable materials.
Environmental and operational norms
Storage conditions: Cool and well-ventilated place (warehouse temperature ≤30℃), away from passageways and high-temperature equipment.
Leakage treatment: For small leaks, use inert materials to adsorb and ventilate. Evacuate the scene in case of a major leak and wear a respirator for handling.
Prohibited behavior: Do not retrieve items that have fallen into the can by hand (long-handled tools must be used). Avoid contact of liquid nitrogen with materials such as plastics and rubber that are prone to brittleness at low temperatures.
Applications
Liquid nitrogen is widely used in various fields, especially where extremely low temperatures are required.
- Cryopreservation:
Liquid nitrogen is extensively used for the ultra-low temperature preservation of biological samples, food, and pharmaceuticals. Its extremely low temperature effectively extends the shelf life of substances, preventing the degradation of biological materials. For instance, in medical research, liquid nitrogen is used to preserve blood, cells, tissues, and other biological samples, ensuring their long-term stability.
- Freeze-drying:
Liquid nitrogen is used in freeze-drying processes to quickly cool and freeze materials, a crucial step in the pharmaceutical and food industries. By rapidly freezing substances, liquid nitrogen helps retain their original structure and properties, effectively preventing loss during the drying process.
- Medical Applications:
Liquid nitrogen is commonly used in cryotherapy, such as the removal of skin lesions (e.g., warts and moles) and the preservation of biological samples (e.g., sperm, eggs, and embryos). Its low-temperature characteristics make it an indispensable tool in these applications.

What is Nitrogen Gas?
Nitrogen gas is the natural gaseous form of nitrogen, omnipresent in our environment. It is typically produced through air separation and plays a vital role in various industrial and scientific research applications.
- Definition:
Nitrogen gas is the natural gaseous form of nitrogen.
- Production:
The production of nitrogen gas usually involves air separation techniques. Common methods include compressing, cooling, and separating the components of air to obtain pure nitrogen gas.
- Physical Properties:
Nitrogen gas is a low-density gas at room temperature. It is a colorless, odorless, non-flammable gas with very stable chemical properties, making it unlikely to react with other substances.
Applications
Nitrogen gas has significant applications in many industrial and research fields.
- Inert Gas:
Nitrogen gas is widely used as an inert gas in industrial processes, such as welding and metal heat treatment, to prevent oxidation and other chemical reactions. In these processes, nitrogen gas provides an inert atmosphere, protecting materials from the effects of oxygen and moisture in the air.
- Chemical Synthesis:
In the chemical industry, nitrogen gas is used to produce ammonia, nitric acid, and other chemicals. It provides an inert environment during reactions, preventing unwanted reactions and enhancing the efficiency and safety of the process.
- Food Packaging:
Nitrogen gas is used in food packaging to prevent oxidation and extend the shelf life of products. By displacing oxygen in packaging, nitrogen gas helps maintain the freshness and flavor of food, making it widely used in packaging snacks, dried fruits, coffee, and other foods.

Key Differences Between Liquid Nitrogen and Nitrogen Gas
Liquid nitrogen and nitrogen gas differ significantly in many aspects, determining their advantages and disadvantages in various applications. Here are some key differences:
Physical State
Liquid nitrogen is the liquid form of nitrogen, while nitrogen gas is its gaseous form. This difference in physical state means they have different properties and uses. Due to its extremely low temperature, liquid nitrogen is often used for rapid cooling and low-temperature preservation. In contrast, nitrogen gas is widely used in industrial processes requiring inert gas protection.
Storage and Transportation
Liquid nitrogen must be stored in specially designed insulated containers to maintain its low temperature. These containers are typically high-vacuum, double-walled structures that effectively insulate and prevent the evaporation of liquid nitrogen. In contrast, nitrogen gas can be stored and transported in high-pressure cylinders, making its storage and transportation more convenient and cost-effective. However, the extremely low temperature of liquid nitrogen requires additional safety measures to prevent frostbite and other hazards.
Cost
The production and storage costs of liquid nitrogen are usually higher than those of nitrogen gas. Producing liquid nitrogen requires significant energy to cool nitrogen gas to a liquid state, resulting in higher costs. Additionally, the storage of liquid nitrogen necessitates special equipment and containers, further increasing costs. In comparison, the production and storage costs of nitrogen gas are lower, making it more economical for many industrial applications.
Safety
Due to its extremely low temperature, liquid nitrogen must be handled with great care. Contact with liquid nitrogen can cause severe frostbite, and it evaporates rapidly, producing large volumes of gas that can create an oxygen-deficient environment. While nitrogen gas does not pose the same low-temperature risks, it must still be handled carefully to avoid high-pressure hazards such as cylinder leaks and explosions.
Advantages and Disadvantages of Liquid Nitrogen and Nitrogen Gas
Liquid nitrogen and nitrogen gas each have unique advantages and disadvantages, essential for selecting the appropriate material and application.
Advantages
- Liquid Nitrogen: Its extremely low temperature makes it irreplaceable in cryopreservation and rapid freezing. It can quickly cool substances, maintaining the long-term stability of biological samples and food.
- Nitrogen Gas: It is cost-effective, easy to store and transport, and widely used as an inert gas and in chemical synthesis. It provides an inert environment, preventing oxidation and contamination.
Disadvantages
- Liquid Nitrogen: It requires high storage and transportation standards and is costly. Maintaining low temperatures requires special equipment and containers, and safety precautions are necessary to prevent frostbite and other hazards.
- Nitrogen Gas: Although cost-effective, it cannot replace liquid nitrogen in some low-temperature applications. Its cooling capacity is insufficient for applications requiring extremely low temperatures.
Conclusion
Liquid nitrogen and nitrogen gas each have significant advantages and specific uses. Liquid nitrogen is irreplaceable in applications requiring ultra-low temperatures, such as cryopreservation and freeze-drying. Nitrogen gas, with its cost-effectiveness and ease of storage and transportation, is widely used in inert gas protection, chemical synthesis, and food packaging. Choosing the right form based on specific needs is crucial to achieving the best results in their respective applications.
If you’re looking for a reliable supplier of nitrogen solutions, look no further than Minnuo. Whether it’s liquid nitrogen or nitrogen gas, Minnuo provides high-quality solutions to ensure your operations run smoothly and efficiently. Contact us today to learn more about how we can support your industrial and research applications.
FAQs
To convert liquid nitrogen to gaseous nitrogen, simply let it warm up to room temperature. When exposed to warmer temperatures, liquid nitrogen naturally evaporates and transitions from its liquid state to a gaseous state. You can control and utilize this process with evaporator equipment, ensuring the efficient and safe conversion of liquid nitrogen to nitrogen gas for various applications.
A nitrogen cylinder contains nitrogen gas. These high-pressure cylinders store and transport nitrogen in its gaseous state, making it convenient for various industrial and research applications. Specially insulated containers maintain the extremely low temperature required to store liquid nitrogen.
The symbol for nitrogen is N, while nitrogen gas is N2. Nitrogen gas consists of two nitrogen atoms forming a diatomic molecule, whereas single nitrogen atoms almost never exist naturally.
Understanding the differences between Liquid Nitrogen and Nitrogen Gas is the first step in optimizing your operations. Minnuo Group specializes in bridging this gap by providing high-efficiency on-site systems that deliver nitrogen in exactly the form you need.
Whether your application requires high-pressure gas for industrial purging or cryogenic liquid for flash freezing, our PSA Nitrogen Generators and Cryogenic Liquefaction Plants provide the perfect solution. We empower businesses to transition from expensive gas deliveries to autonomous, on-site production, cutting logistics costs by up to 30%.
Why Partner with Minnuo Group?
- Flexible Delivery: Custom-engineered systems designed for seamless switching between gaseous and liquid states.
- Ultra-High Purity: Stable output of up to 99.999% purity, meeting the rigorous standards of the electronics and pharmaceutical industries.
- Reliable Support: 24/7 technical assistance and global on-site service to ensure your gas supply never stops.
Optimize your nitrogen supply today. Contact Minnuo Group for professional technical advice and a competitive quote!






 sales2:+86 17506119168
sales2:+86 17506119168

