In the operation and application of nitrogen generators, a deep understanding of the units and conversion methods of common parameters in nitrogen generators is crucial for accurately controlling the performance and operational efficiency of the equipment. These parameters not only reflect the physical state of the gas but also serve as an indispensable theoretical basis in the design, operation, and maintenance of nitrogen generators.
Common parameters in nitrogen generators include mass, volume, specific volume, pressure, temperature, flow rate, and purity, involving various units and conversion methods. Mass is expressed in milligrams, grams, kilograms, and tons. Volume is measured in cubic millimeters, cubic centimeters, and cubic meters. Pressure units include pascals, kilopascals, and megapascals. Temperature is measured in degrees Celsius and Kelvin. Flow rate is divided into volume flow rate and mass flow rate, and purity can be classified into industrial nitrogen, pure nitrogen, and high – purity nitrogen.

I. Mass, Volume, and Specific Volume
(I) Mass
As an inherent property of gas, mass is a physical quantity possessed by the aggregate of gas molecules. Its commonly used units are derived from the basic units of the International System of Units, including:
- Milligram (mg)
- Gram (g)
- Kilogram (kg)
- Ton (t)
It is commonly used to represent the mass of gases and other substances. The conversion relationships are as follows:
- 1 ton = 1000 kg
- 1 kg = 1000 g
- 1 g = 1000 mg
(II) Volume
Volume is used to quantify the space occupied by gas. Commonly used units are:
- Cubic millimeter (mm³)
- Cubic centimeter (cm³)
- Cubic meter (m³)
It can be used to quantify the volume of gas occupied in space. The conversion relationships are as follows:
- 1 m³ = 1,000,000 cm³
- 1 cm³ = 1000 mm³
(III) Specific Volume
According to the definition of thermodynamics, specific volume is the volume occupied by a unit mass of a substance, denoted by the symbol V. In the gaseous state, its unit is m³/kg; in the liquid state, the unit is l/kg.
In the calculation of the gas quantity in a nitrogen generator, based on the ideal gas law:
pV = nRT
(where p is pressure, V is volume, n is the amount of substance, R is the gas constant, and T is temperature)
Combined with the gas mass and specific volume, the volume of the storage container required can be accurately calculated, which is of great significance in equipment selection and engineering design.
II. Pressure – related Parameters
(I) Pressure and Pressure Intensity
1. Micro – principle
At the micro level, gas pressure results from the continuous impact of gas molecules in random thermal motion on the container wall. The force of this impact per unit area is the pressure intensity.
2. Units
- Traditional units: In the traditional measurement system, millimeters of mercury (mmHg) are commonly used.
- International units: In the International System of Units (SI), the legal units of measurement are pascal (Pa), kilopascal (kPa), and megapascal (MPa).
3. Conversion relationships
Based on the principles of fluid statics, the following conversion relationships can be derived:
- 1mmHg = 133.3Pa = 0.1333kPa
- 1MPa = 1000kPa = 1000000Pa
- 1ATA = 0.1MPa
During the operation of a nitrogen generator, the gas transportation efficiency and the equipment’s pressure – resistance requirements are closely related to the pressure parameters. Excessively high or low pressure can affect the normal operation of the nitrogen generator.
(II) Atmospheric Pressure, Absolute Pressure, and Relative Pressure
1. Definitions
- Atmospheric pressure: The Earth’s surface is surrounded by a thick atmosphere. The pressure exerted by the atmosphere on the Earth’s surface or objects on the surface is defined as “atmospheric pressure,” denoted by the symbol B.
- Absolute pressure: Absolute pressure is the pressure directly acting on the surface of a container or object, with absolute vacuum as the zero point, denoted as PABS.
- Relative pressure: The pressure measured by pressure – measuring instruments such as pressure gauges, vacuum gauges, and U – tubes is the “gauge pressure” (also known as relative pressure) based on atmospheric pressure, denoted by the symbol Pg.
2. Relationships
According to the principle of pressure superposition, the following relationship exists among the three:
PABS = B + Pg
In the pressure monitoring and control system of a nitrogen generator, clearly distinguishing these three pressure concepts is crucial for ensuring the safe and stable operation of the equipment and avoiding equipment failures and safety accidents caused by abnormal pressure.
III. Temperature – related Parameters
(I) Temperature and Absolute Temperature
1. Micro – essence
At the micro level, temperature reflects the intensity of the thermal motion of matter molecules and is the statistical average of molecular thermal motion. The temperature of gas also stems from the thermal motion of gas molecules.
2. Common temperature scales
- Celsius scale: In daily life and engineering fields, the Celsius scale (℃) is commonly used to represent temperature, with the freezing point of water set at 0℃.
- Absolute scale: In physics research, the absolute scale (K) is more widely used, with absolute zero (- 273.15℃) as the zero point. The conversion relationship between Celsius temperature and absolute temperature is:
T = t + 273.15
- Fahrenheit scale: In some research and applications of the imperial unit system, the Fahrenheit scale (℉) is also used.
3. Application significance
In the cooling and heating processes of a nitrogen generator, based on the principle of heat exchange and the gas state equation, precise temperature control is crucial for maintaining the stable operation of the nitrogen generator and ensuring the quality and output of nitrogen.
(II) Critical Temperature and Critical Pressure
1. Phase – change theory
According to the gas – liquid phase – change theory, any gas can be liquefied under specific temperature and pressure conditions. As the temperature rises, the thermal motion of gas molecules intensifies, and the pressure required for liquefaction also increases.
2. Definitions
When the temperature exceeds a certain specific value, no matter how much pressure is applied, the gas cannot be liquefied. This temperature is called the critical temperature, and the minimum pressure required for liquefaction at this temperature is the critical pressure.
3. Process value
In the nitrogen – making process, a deep understanding of the critical parameters of gas helps to optimize gas liquefaction and separation operations, improving nitrogen – making efficiency and product quality.
(III) Dew Point
1. Definition
The dew point refers to the temperature at which water vapor in a gas changes from an unsaturated state to a saturated state under a certain pressure. When the dew – point temperature is reached, water vapor begins to condense into small dew droplets.
2. Classification
Since the dew point is closely related to pressure, there is a distinction between atmospheric dew point (normal pressure dew point) and dew point under pressure.
- Atmospheric dew point: It refers to the condensation temperature of water vapor under standard atmospheric pressure.
- Dew point under pressure: It is the condensation temperature corresponding to a specific pressure.
3. Conversion and application
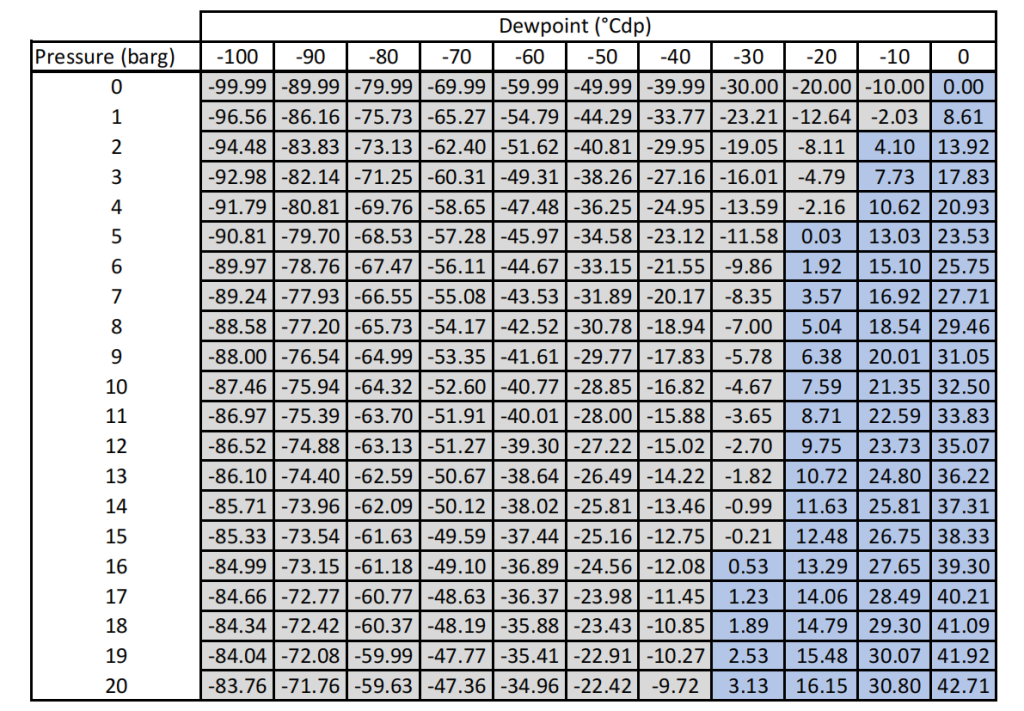
There is a specific conversion relationship between the two, which can be obtained by referring to professional dew – point conversion tables. In the gas – drying process of a nitrogen generator, strictly controlling the dew point can effectively prevent the impact of moisture on the quality of nitrogen, ensuring that nitrogen meets the application requirements of different industries.
Here is a dew – point conversion table for you.
IV. Flow – related Parameters
(I) Definitions and Classifications
1. Definition
Flow rate is an important parameter describing the flow characteristics of gas in a pipeline, defined as the amount of gas passing through any cross – section per unit time during the gas – flow process.
2. Classifications
Flow rate can be expressed in two ways: volume flow rate and mass flow rate.
- Volume flow rate: It refers to the volume of gas passing through any cross – section of a pipeline per unit time.
- Mass flow rate: It refers to the mass of gas passing through per unit time.
(II) Units and Standard Conditions
1. Commonly used units
In the gas industry, the commonly used unit of volume flow rate is m³/h (or L/H).
2. Standard conditions
Considering that the volume of gas is significantly affected by temperature, pressure, and humidity, for the convenience of unified comparison and measurement, the volume flow rate usually refers to the flow rate under standard conditions (temperature is 20℃, pressure is 0.101MPa, and relative humidity is 65%), with the unit of Nm³/h, where “N” represents standard conditions.
3. Key to application
The flow – rate parameter directly determines the gas – production speed of the nitrogen generator. Different production processes and application scenarios have different flow – rate requirements for the nitrogen generator. Reasonably selecting and regulating the flow – rate parameter is the key to meeting diverse production needs.
V. Purity
(I) Grade Classification
As a key technical indicator for measuring gas quality, for nitrogen, according to national standards, its purity can be divided into three grades:
- Industrial nitrogen: The purity is 99.5% (O₂ content is less than or equal to 0.5%).
- Pure nitrogen: The purity reaches 99.99% (O₂ content is less than or equal to 0.01%).
- High – purity nitrogen: The purity is as high as 99.999% (O₂ content is less than or equal to 0.001%).The content of other impurities is also strictly regulated.
(II) Differences in Industry Requirements
The requirements for nitrogen purity vary significantly among different industries:
- Electronics industry: In high – precision processes such as chip manufacturing, high – purity nitrogen is often required to ensure product quality.
- Chemical industry: In some general reactions, the requirement for nitrogen purity is relatively low.
VI. Other Important Parameters and Supplementary Conversions
(I) Concentration
1. Representation method
In the nitrogen – making process, concentration is closely related to purity and is usually expressed as a percentage(Unit: %).
2. Calculation significance
Taking a gas mixture as an example, the nitrogen concentration refers to the volume fraction or mole fraction of nitrogen in the gas mixture, which is inversely proportional to the content of other gases. When calculating and analyzing the gas composition produced by a nitrogen generator using methods such as gas chromatography, the concentration parameter is a key analysis indicator.
(II) Molar Volume
1. Definition under standard conditions
Under standard conditions (STP, i.e., temperature is 0℃, pressure is 101.325kPa), according to Avogadro’s law, the volume occupied by 1 mole of any ideal gas is approximately 22.4L.
2. Conversion application
With the concept of molar volume, the conversion between the amount of substance and gas volume can be achieved. In the nitrogen – making process, by calculating the amount of substance of the air used as the raw material for nitrogen production, the nitrogen – making process parameters can be optimized, improving the utilization rate of raw materials.
(III) Gas Constant Conversion
1. Differences in unit systems
The gas constant has different values in different unit systems. In the International System of Units, the universal gas constant R = 8.314 J/(mol·K). In the imperial unit system, the value of the gas constant is different.
2. Key points of application
When it comes to international cooperation or the design and calculation of nitrogen generators based on different standards, accurate conversion of the gas constant is necessary to ensure the accuracy and reliability of the calculation results.

Ⅶ.Conclusion
In the field of industrial gas production, nitrogen generators play a crucial role. Mastering the common parameters in nitrogen generators, such as pressure, flow rate, purity, etc., as well as understanding the unit conversions between them, is not just a theoretical necessity but a key factor in ensuring the efficient operation of nitrogen generators and producing high-quality nitrogen that meets the diverse needs of various industries.
With the continuous advancement of technology, nitrogen generation technology is evolving at an unprecedented pace. In the future, the research dimensions of these parameters will continue to expand, and the application scenarios will keep diversifying.
As a professional manufacturer deeply involved in the gas equipment field, MINNUO is always dedicated to providing cutting-edge nitrogen generation solutions for its clients. If you have any questions regarding nitrogen generator knowledge, equipment selection, or technical details, feel free to reach out to us. We are always here to assist you.






 sales2:+86 17506119168
sales2:+86 17506119168

